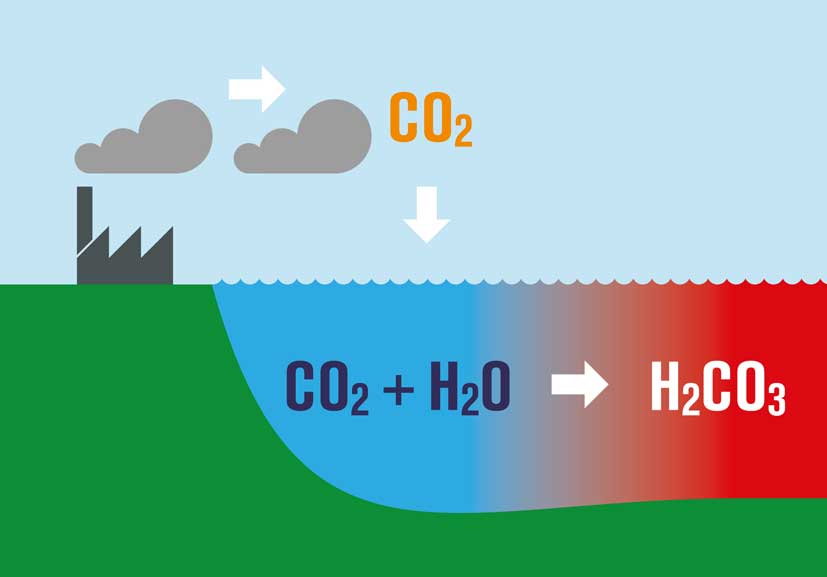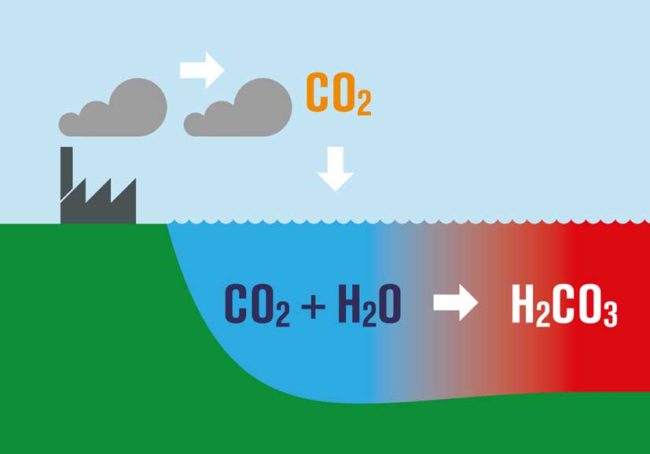
What is perhaps not appreciated by some is that as CO2 increases and the oceans continue to absorb it, there is a huge consequence. The basic chemistry involved results in what is known as Ocean Acidification.
There is a paper that was published back in 2008 that examined the impact that various level of CO2 within our atmosphere would have upon the oceans. Entitled “Atmospheric CO2 stabilization and ocean acidification”, the full text is available online without any paywall.
First, let’s look at the basic chemistry
- As we burn fossil fuels, we emit CO2
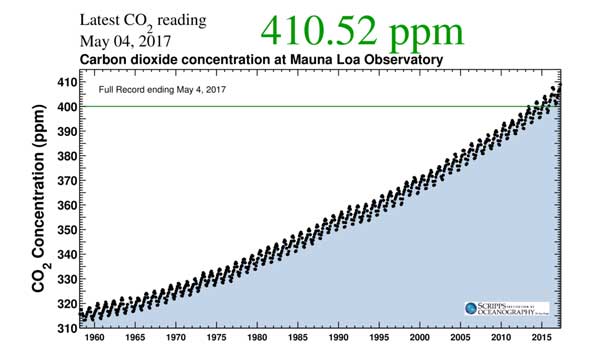
- The levels of CO2 measured within our atmosphere have been increasing. It has climbed from 280ppm in 1700 to 410ppm and is still increasing
- About 45% of what we emit stays within the atmosphere, some if taken up by plants, but the rest is absorbed by the oceans.
Dissolving CO2 in seawater increases the hydrogen ion (H+) concentration in the ocean, and thus decreases ocean pH, as follows:
So far, ocean pH has dropped from 8.2 to 8.1 since the industrial revolution, and is expected by fall another 0.3 to 0.4 pH units by the end of the century.
A drop in pH of 0.1 might not seem like a lot, but the pH scale, like the Richter scale for measuring earthquakes, is logarithmic. For example, pH 4 is ten times more acidic than pH 5 and 100 times (10 times 10) more acidic than pH 6.
If we continue to add carbon dioxide at current rates, seawater pH may drop another 120 percent by the end of this century, to 7.8 or 7.7, creating an ocean more acidic than any seen for the past 20 million years or more.
In essence, it will have a huge impact upon life in the ocean. Some will adopt, and much of it will not.
Various Scenarios
The 2008 paper used a model to examine the question “What happens to Ocean Acidification if we stabilise CO2 at various different levels?”. The results are not good …
Our simulations show the potential for major damage to at least some ocean ecosystems at atmospheric CO2 stabilization levels as low as 450 ppm.
Before the industrial revolution, more than 98% of corals reefs were surrounded by waters that were >3.5 times saturated with respect to their skeleton materials (aragonite). If atmospheric CO2 is stabilized at 450 ppm only 8% of existing coral reefs will be surrounded by water with this saturation level. Also at this CO2 level 7% of the ocean South of 60°S will become undersaturated with respect to aragonite, and parts of the high latitude ocean will experience a decrease in pH by more than 0.2 units.
Remember that we have already reached 410ppm, so the 450ppm level is not very far away.
Bottom Line
It is now generally appreciated that increasing atmospheric CO2 concentrations interferes with our climate system by trapping infrared radiation. What is not so well appreciated is that increasing CO2 also affects marine ecosystems by perturbing ocean chemistry.
Further Reading
- Wikipedia Article on Ocean Acidification
- UK Ocean Acidification Research Programme
- Smithsonian Ocean Portal Acidification page
Tweets
If CO2 exceeds ~450 ppm,very bad for ocean acidification (https://t.co/LUjn29ziEd).
Further justification for 450ppm/2C as "safe" limit…— Michael E. Mann (@MichaelEMann) July 23, 2017
Our best available understanding indicates that ocean carbon uptake increases substantially as emissions drop: https://t.co/PXRwf9tohE
— Michael E. Mann (@MichaelEMann) July 23, 2017
https://twitter.com/MMarker1992/status/889246525544681472